Release of our first CSR Report!
We’re excited to announce the publication of our first CSR Report!
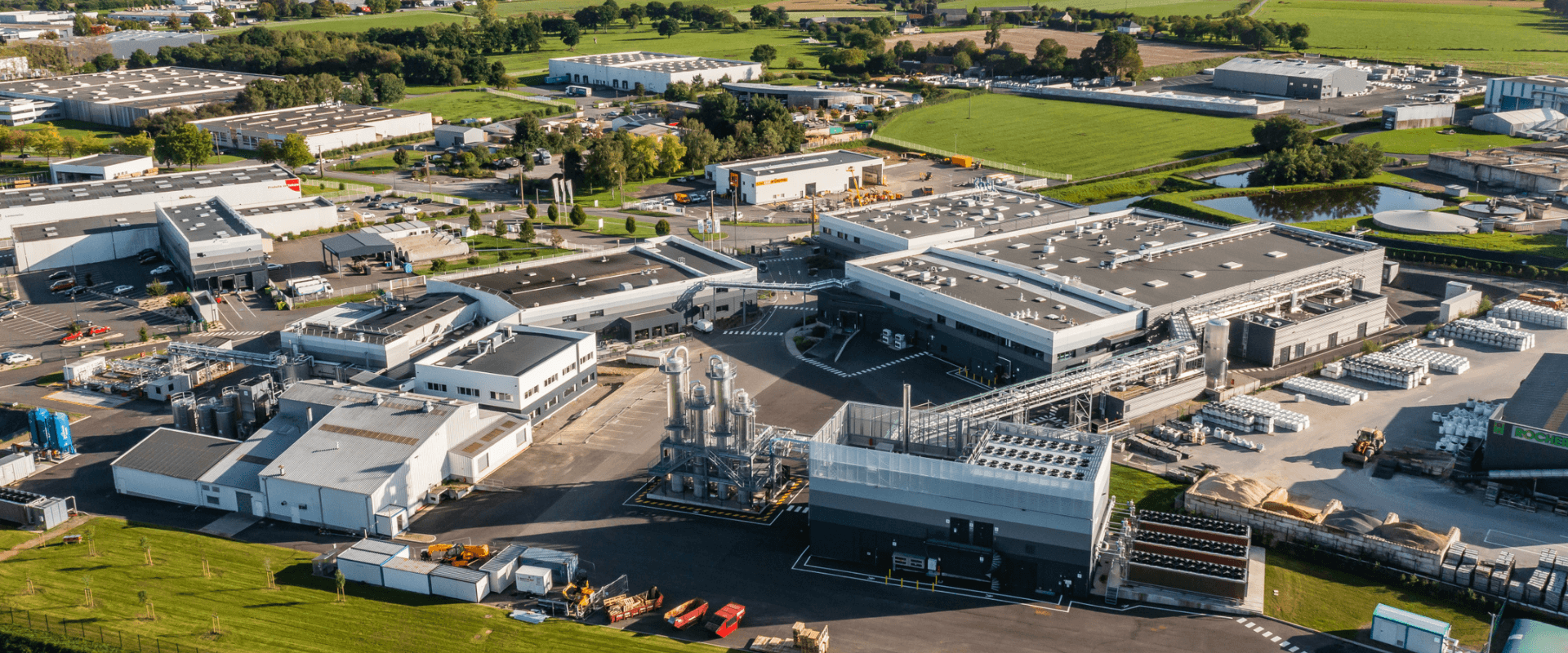
Located in Javené, France our production site of 64 000 square meters host state of the art facilities :


HTL Biotechnology has developed industrial facilities to secure supplies for its customers, while meeting growing demand for both hyaluronic acid and DNA :
Further investments are in progress to increase our DNA and Hyaluronic Acid production capacity over the coming years.
Quality is our identity :
Our expertise developping new biopolymer and functionalizing Hyaluronic Acid is supported by :


Our laboratory is equiped to perform different kind of analysis :
Thanks to our qualified equipment, certified larboratory technicians and validated methods, we ensure a reliable quality control of our products.
Our Quality Control laboratory ensures our products meet the specifications of clients.